What Is The Half Titration Point . When this happens, the concentration of h + ions equals the k a value of the acid. the half equivalence point, also known as the midpoint, is a significant point in a titration that occurs when half of the. one point in the titration of a weak acid or a weak base is particularly important: if you are titrating an acid against a base, the half equivalence point will be the point at which half the acid has.
from www.numerade.com
the half equivalence point, also known as the midpoint, is a significant point in a titration that occurs when half of the. When this happens, the concentration of h + ions equals the k a value of the acid. one point in the titration of a weak acid or a weak base is particularly important: if you are titrating an acid against a base, the half equivalence point will be the point at which half the acid has.
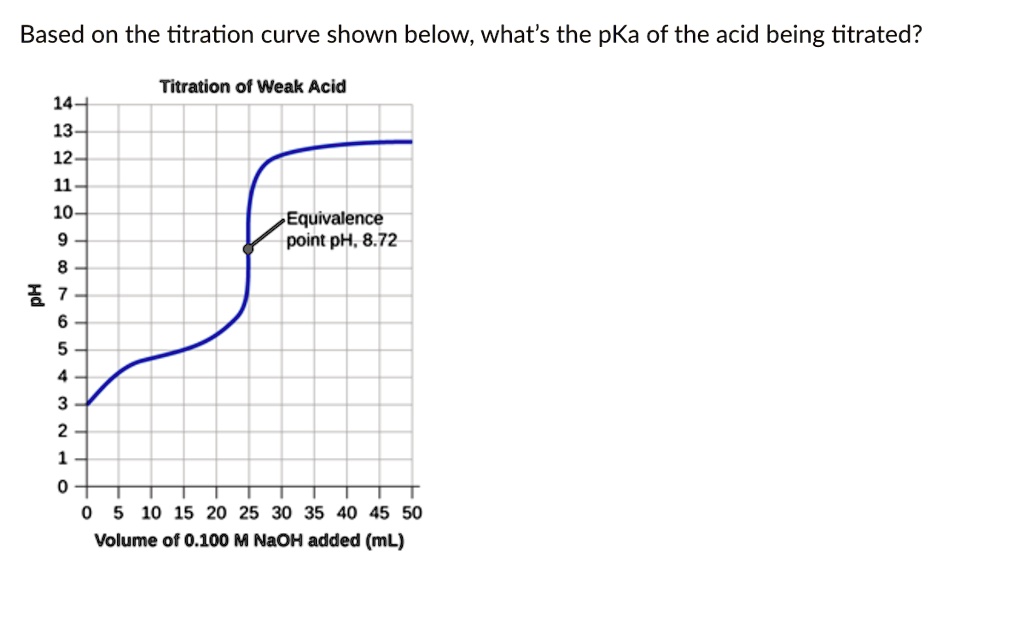
Based on the titration curve shown below, what's the pKa of the acid
What Is The Half Titration Point the half equivalence point, also known as the midpoint, is a significant point in a titration that occurs when half of the. if you are titrating an acid against a base, the half equivalence point will be the point at which half the acid has. When this happens, the concentration of h + ions equals the k a value of the acid. one point in the titration of a weak acid or a weak base is particularly important: the half equivalence point, also known as the midpoint, is a significant point in a titration that occurs when half of the.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts What Is The Half Titration Point one point in the titration of a weak acid or a weak base is particularly important: the half equivalence point, also known as the midpoint, is a significant point in a titration that occurs when half of the. When this happens, the concentration of h + ions equals the k a value of the acid. if you. What Is The Half Titration Point.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps What Is The Half Titration Point if you are titrating an acid against a base, the half equivalence point will be the point at which half the acid has. the half equivalence point, also known as the midpoint, is a significant point in a titration that occurs when half of the. one point in the titration of a weak acid or a weak. What Is The Half Titration Point.
From www.vrogue.co
Acide Base Titration The Half Equivalence Point vrogue.co What Is The Half Titration Point one point in the titration of a weak acid or a weak base is particularly important: When this happens, the concentration of h + ions equals the k a value of the acid. the half equivalence point, also known as the midpoint, is a significant point in a titration that occurs when half of the. if you. What Is The Half Titration Point.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent What Is The Half Titration Point one point in the titration of a weak acid or a weak base is particularly important: if you are titrating an acid against a base, the half equivalence point will be the point at which half the acid has. the half equivalence point, also known as the midpoint, is a significant point in a titration that occurs. What Is The Half Titration Point.
From www.chegg.com
Solved Equivalence Point pH = 8.96 All acid converted to What Is The Half Titration Point When this happens, the concentration of h + ions equals the k a value of the acid. one point in the titration of a weak acid or a weak base is particularly important: if you are titrating an acid against a base, the half equivalence point will be the point at which half the acid has. the. What Is The Half Titration Point.
From dxomjwenv.blob.core.windows.net
Titration Half Equivalence Point at Karen Winkleman blog What Is The Half Titration Point one point in the titration of a weak acid or a weak base is particularly important: the half equivalence point, also known as the midpoint, is a significant point in a titration that occurs when half of the. When this happens, the concentration of h + ions equals the k a value of the acid. if you. What Is The Half Titration Point.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe What Is The Half Titration Point When this happens, the concentration of h + ions equals the k a value of the acid. one point in the titration of a weak acid or a weak base is particularly important: the half equivalence point, also known as the midpoint, is a significant point in a titration that occurs when half of the. if you. What Is The Half Titration Point.
From www.chegg.com
Solved The halfequivalence point of a titration occurs half What Is The Half Titration Point if you are titrating an acid against a base, the half equivalence point will be the point at which half the acid has. one point in the titration of a weak acid or a weak base is particularly important: the half equivalence point, also known as the midpoint, is a significant point in a titration that occurs. What Is The Half Titration Point.
From www.chegg.com
Solved The halfequivalence point of a titration occurs half What Is The Half Titration Point one point in the titration of a weak acid or a weak base is particularly important: the half equivalence point, also known as the midpoint, is a significant point in a titration that occurs when half of the. if you are titrating an acid against a base, the half equivalence point will be the point at which. What Is The Half Titration Point.
From dxomjwenv.blob.core.windows.net
Titration Half Equivalence Point at Karen Winkleman blog What Is The Half Titration Point if you are titrating an acid against a base, the half equivalence point will be the point at which half the acid has. the half equivalence point, also known as the midpoint, is a significant point in a titration that occurs when half of the. When this happens, the concentration of h + ions equals the k a. What Is The Half Titration Point.
From www.differencebetween.com
Difference Between AcidBase Titration and Redox Titration Compare What Is The Half Titration Point the half equivalence point, also known as the midpoint, is a significant point in a titration that occurs when half of the. When this happens, the concentration of h + ions equals the k a value of the acid. if you are titrating an acid against a base, the half equivalence point will be the point at which. What Is The Half Titration Point.
From www.numerade.com
Based on the titration curve shown below, what's the pKa of the acid What Is The Half Titration Point the half equivalence point, also known as the midpoint, is a significant point in a titration that occurs when half of the. When this happens, the concentration of h + ions equals the k a value of the acid. if you are titrating an acid against a base, the half equivalence point will be the point at which. What Is The Half Titration Point.
From srkzhxhdjshcc.blogspot.com
How To Find Half Equivalence Point On Titration Curve Excel Is there What Is The Half Titration Point When this happens, the concentration of h + ions equals the k a value of the acid. one point in the titration of a weak acid or a weak base is particularly important: if you are titrating an acid against a base, the half equivalence point will be the point at which half the acid has. the. What Is The Half Titration Point.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint What Is The Half Titration Point When this happens, the concentration of h + ions equals the k a value of the acid. the half equivalence point, also known as the midpoint, is a significant point in a titration that occurs when half of the. one point in the titration of a weak acid or a weak base is particularly important: if you. What Is The Half Titration Point.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co What Is The Half Titration Point one point in the titration of a weak acid or a weak base is particularly important: if you are titrating an acid against a base, the half equivalence point will be the point at which half the acid has. the half equivalence point, also known as the midpoint, is a significant point in a titration that occurs. What Is The Half Titration Point.
From dxobegonq.blob.core.windows.net
Titration Curve Labels at Ok Moore blog What Is The Half Titration Point the half equivalence point, also known as the midpoint, is a significant point in a titration that occurs when half of the. if you are titrating an acid against a base, the half equivalence point will be the point at which half the acid has. When this happens, the concentration of h + ions equals the k a. What Is The Half Titration Point.
From www.chegg.com
Solved The halfequivalence point of a titration occurs half What Is The Half Titration Point When this happens, the concentration of h + ions equals the k a value of the acid. one point in the titration of a weak acid or a weak base is particularly important: the half equivalence point, also known as the midpoint, is a significant point in a titration that occurs when half of the. if you. What Is The Half Titration Point.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free What Is The Half Titration Point the half equivalence point, also known as the midpoint, is a significant point in a titration that occurs when half of the. one point in the titration of a weak acid or a weak base is particularly important: When this happens, the concentration of h + ions equals the k a value of the acid. if you. What Is The Half Titration Point.